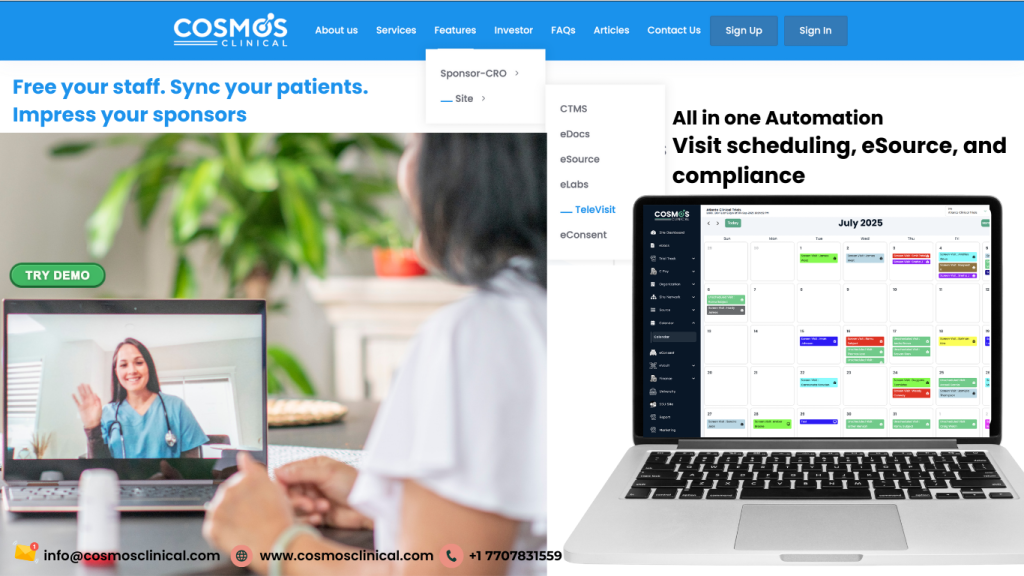
Introduction to Clinical Trial Efficiency
Clinical trials are becoming increasingly complex, with tight timelines, diverse patient populations, and regulatory requirements. One of the biggest challenges sites face is efficiently managing visit schedules while maintaining accurate data and ensuring compliance. Fortunately, technology can turn these challenges into opportunities for efficiency and quality.
Smart Visit Scheduling for Clinical Trials
Coordinating patient visits across multiple studies can be chaotic. Traditional scheduling methods often result in missed visits, delays, or staff overload. With COSMOS Clinical, sites can implement smart visit scheduling with automated reminders for both staff and patients. This not only reduces administrative burden but also improves patient retention and adherence.
eSource Automation and Data Capture
Manual data entry is prone to errors and consumes valuable time. COSMOS Clinical’s built-in eSource automation allows data to be captured directly during patient visits. This ensures accuracy, reduces transcription errors, and frees staff to focus on patient care and critical study tasks.
Ensuring Regulatory Compliance and GCP Standards
Regulatory compliance is non-negotiable in clinical research. COSMOS Clinical helps sites stay audit-ready with automated documentation, real-time tracking, and secure storage of trial data. This reduces the risk of deviations and ensures adherence to GCP standards.
Conclusion: Improving Site Management with Technology
By leveraging technology to streamline visit scheduling, automate data capture, and strengthen compliance, clinical research sites can boost efficiency, enhance data quality, and focus on what truly matters—delivering high-quality patient care and research outcomes.
Call to Action
If your site is looking to optimize workflows and take your clinical trials to the next level, COSMOS Clinical can make it happen.
info@cosmosclinical.com
+1 7707831559