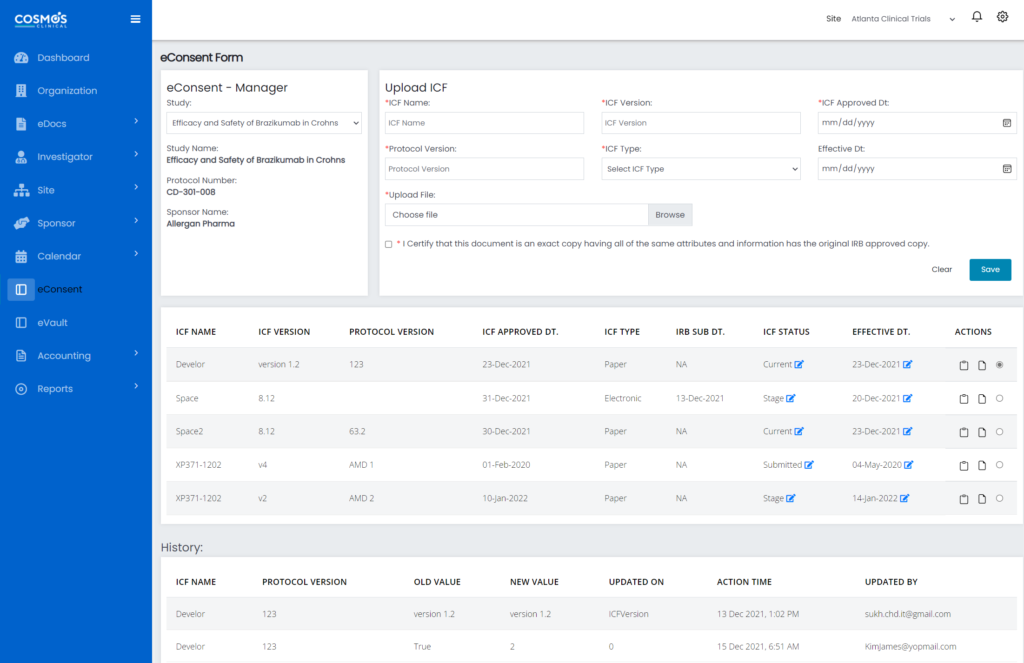
COSMOS eConsent transforms the conventional patient consent approach by making the entire process easy, truly patient-centric, and improving transparency. It is designed for decentralized, virtual, and hybrid research projects to help deliver compliant, patient-centric, and site-friendly experiences. Through our digital consent solution, you can offer patients the much-needed flexibility for remote consenting.
Our innovative, regulatory-compliant, and patient-friendly eConsent system automates the entire patient enrolment process. This improves the overall patient consent tracking management, reduces informed consent errors, and eases the administrative burden in research sites and among study teams. Also, it improves the patient experience by granting patients access to easy-to-understand clinical trial information and facilitating patient engagement.
Features
- Traceable and fully compliant with US 21 CFR Part 11 electronic signature requirements
- Maximize patient’s clinical trial comprehension with diagrams, images, videos, and screen readers for accessibility
- Increase patient engagement and retention by ensuring critical information is always available at the participants’ fingertips.
- COSMOS built-in video conferencing capability keeps you focused on participants and offers seamless patient enrollment and engagement experience
- Conduct secure (encrypted) peer-to-peer video conversations with industry-leading platform
- Build unprecedented confidence and assurance for both patients and study teams in the new remote clinical trial space
- Empower patients and research sites with an eConsent solution that improves education, retention, and adherence to the regulatory requirements
- COSMOS delivers device-independent compliant, efficient, flexible eConsent workflow solution
Participant safety and data integrity are the most important factors
Increased Transparency and Compliance
All consent records are stored in an encrypted cloud platform with tools for version-controlled file storage, deployment, approval, and strict monitoring. This facilitates a higher level of transparency and compliance across your clinical study patient enrollment and retention. Note that both single and dual in-line signature eConsent/TeleVisit solutions are available with full compliance, audit trails, and reporting.
Enhanced Informed Consent
Principal Investigators (PIs) and research site teams can use COSMOS eConsent to send ICFs and engaging educational content to the patient. This content explains the critical and complex terms to ensure optimal understanding of the clinical study and commitments, allowing patients to make informed enrollment choices. With eConsent, PIs and site teams can remotely make TeleVisit and patient phone calls more effective.
With COSMOS eConsent, the potential study participant receives the documents electronically, reviews them in real-time with the PI or site team, and provides their in-line digital signature. Once the document is signed, it will automatically be available for countersigning, and a digital copy will be sent to the participant electronically.
Flexibility and Patient Experience
COSMOS eConsent is designed to integrate seamlessly with the site’s existing infrastructure, allowing sites to deploy the solution efficiently and quickly. It is also accessible for different device types, offering patients the flexibility to access engaging and interactive multimedia to help them make informed choices.

Optimize Patient Enrollment Workflows for centralized and decentralized or virtual trials
- Empower sites and patients with an eConsent solution that improves patient education and enrollment compliance
- Provide simple, intuitive consent experience and the flexibility patients need to offer informed consent remotely
- Deploy tailored consent experiences that improve knowledge transfer through secure video conversations
- Facilitate study-specific e-signatory workflows that create flexibility for PIs, site teams, and study participants.