COSMOS Clinical Trial Management System (CTMS ) allows you to design a clinical study, maximize insights and manage clinical trials effortlessly. This premier clinical trial management system can be configured to address various challenges associated with clinical trials management. It improves user accessibility, streamlines communication, reduces manual operations, and eliminates redundant tasks to facilitate more efficient clinical trials.
Improve engagement, participation, and retention
Use various tools to streamline patient enrollment, improve patient engagement and retention. Stay in contact with the patients using alerts to minimize no-show appointments and the overall engagement in your clinical study.
Consistency is key
COSMOS’ powerful site and investigator database expedite the process of aligning one or more study sites with GCP, 21 CFR Part 11, and other compliance requirements to guarantee safe and high-quality clinical research.
Integrate your Calendar for Seamless Scheduling
Automate your study target dates through calendar integration and setting reminders. You can seamlessly integrate departmental, individual, and company calendars for adequate scheduling and visibility—set alerts and notifications for critical tasks and follow-ups. You can also view the upcoming visits, overdue visits, and all pending actions.
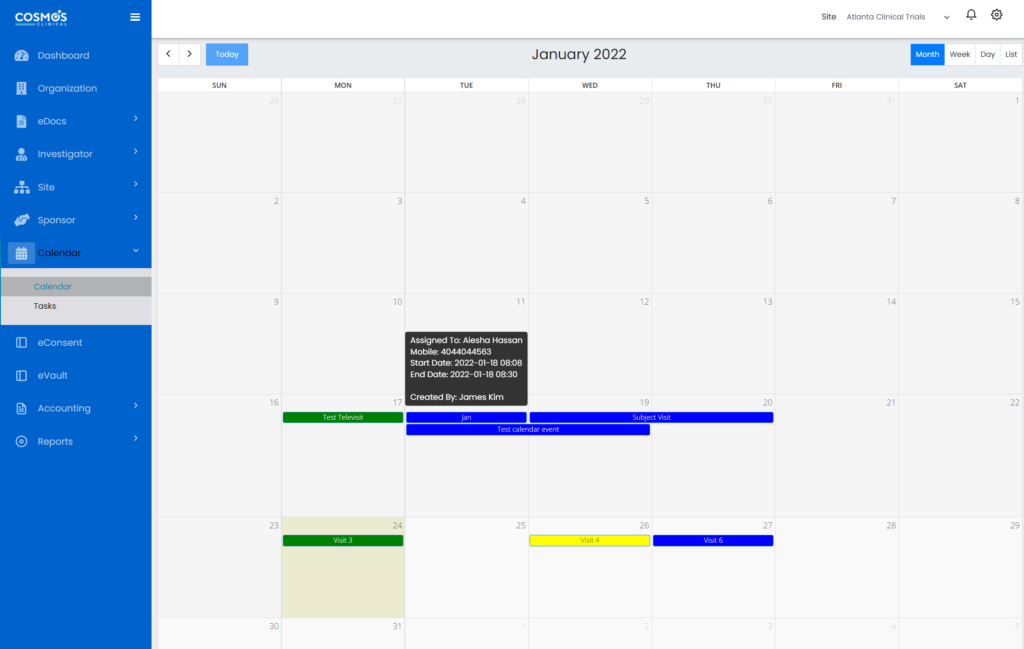
Manage the Subject Database on the Study Site
Automatically import and sort database social media, website, and email campaign leads. Tailor each advertising campaign to the corresponding clinical research. Finally, take advantage of the CTMS’ automated messages, emails that may be pre-screened and sent. All these features are available in a single, easy-to-use subject lead funnel.
Document Repository on the Main study Site
Connect all your site’s clinical operations and link every trial to its corresponding content repository. Enjoy unlimited access to a dashboard with oversight and visualizations on milestones, enrollment, documents, activities, and clinical research plans in a given site.
Use Text Messages for Integration
COSMOS CTMS utilizes a two-way SMS feature to increase patient retention by sending reminders to users remotely to optimize recruitment and enrollment.
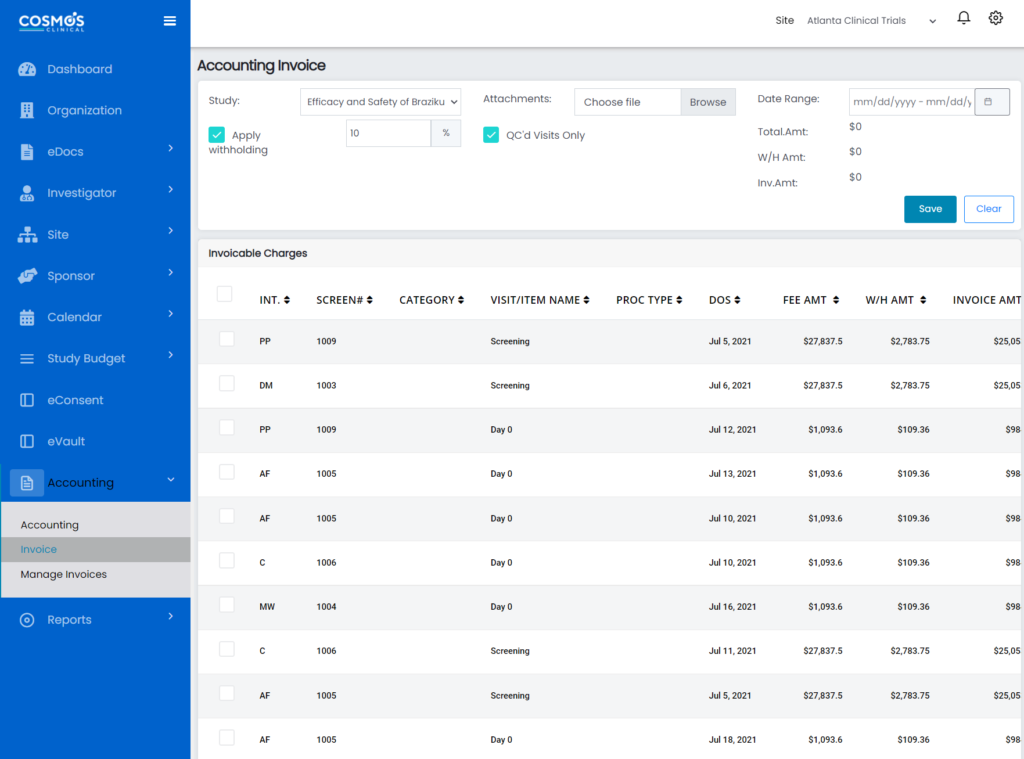
Understand the State of your Finances
- Automate the creation and analysis of earnings, expenses, receivables, and billable reports for visit and study level financial activities.
- Use the interactive financial overview dashboard to organize and automate your financial information reports and avoid missed billing.
- Our CTMS facilitates dynamic financial report tracking and real-time bill reconciliation.
- Track credentials & billing compliance to avoid costly deviations.
Clinical Trials Management System for Next-Generation Studies.
Request a demo to know more about COSMOS CTMS !