COSMOS eSource: Electronic Clinical Source Data Collection
Clinical research highly depends on precision. Imagine teams wasting hours deciphering handwritten notes instead of analyzing results—or a typo in a document derailing months of work. Manual forms aren’t just slow—they’re prone for human error. A single data entry mistake can change the results, forcing teams into back-and-forth data corrections. Meanwhile, staff spends time doing data corrections, budgets bleed, and sometimes, it creates unreliable data. It’s a recipe for wasted time, soaring costs, and risks no one can afford. Moreover, adherence to ALCOA-C principles—ensuring data is Attributed, Legitimate, Contemporaneous, Original, Accurate, and Complete—demands a meticulous approach that manual processes struggle to fulfill.
Increase Research Site Efficiency
Ensure compliance and minimize protocol deviations in your research site by setting data parameters, establishing the necessary fields for data collection, and assigning read-only privileges for QA/QC users. In fact, you can create a source document quickly using COSMOS pre-built source template library. Our user-friendly interface makes it easy to integrate appointment calendars, create processes, and assign them. That means you can design a clinical study within a few hours.
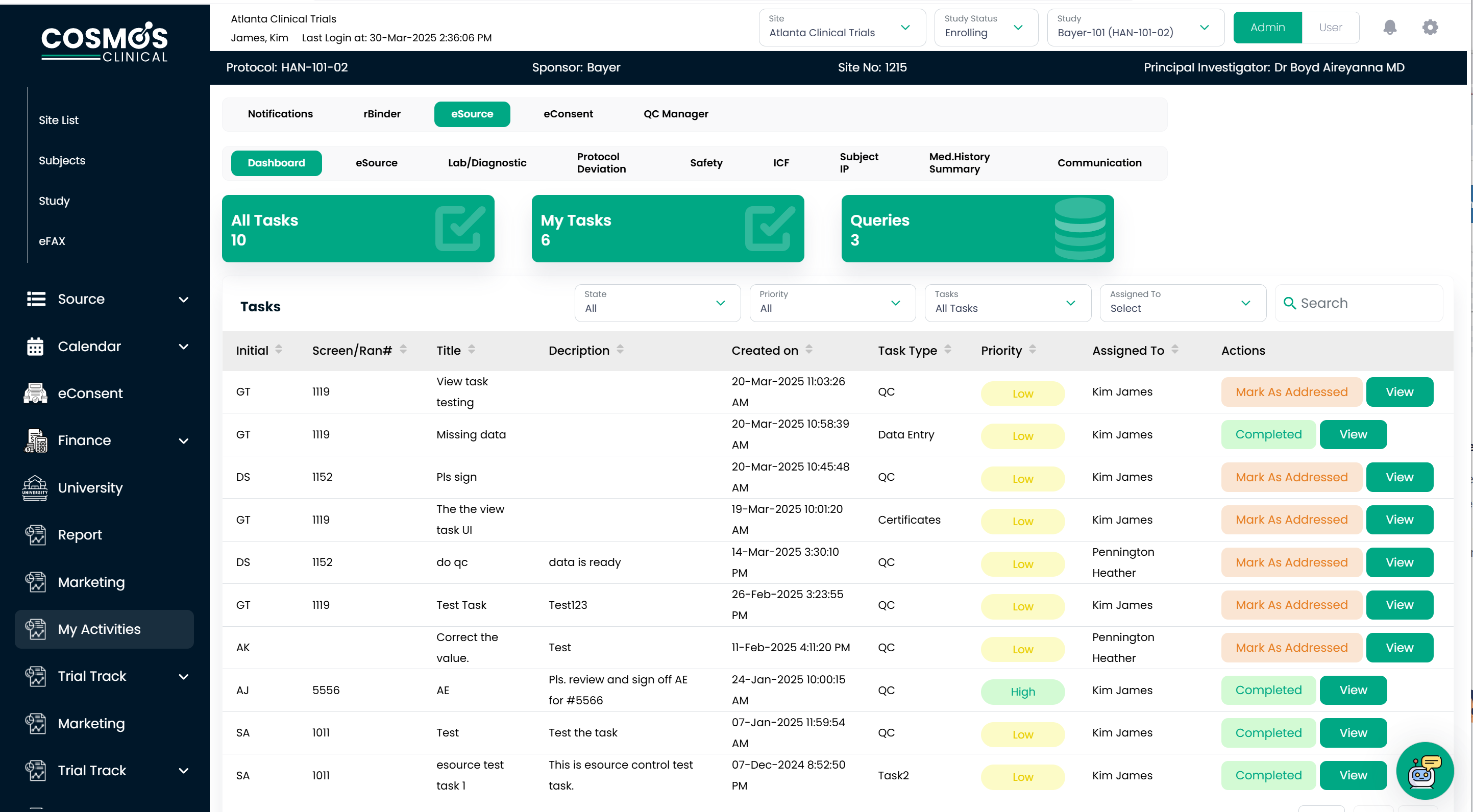
Data Revolution: Smarter Insights, Faster Outcomes
Manual data transcription is not just time-intensive—it is risky and error-prone. Transferring handwritten data into Electronic Data Capture (EDC) systems is prone to mistakes that trigger EDC queries. This inefficiency forces site teams and sponsor monitors into a back-and-forth data validation, delaying database lock and impacting study timelines. COSMOS eSource reduces these roadblocks by facilitating real-time data capture, dramatically reducing the need for multiple data corrections and unnecessary monitoring visits.
As an integrated platform, it ensures that data, once entered, flows seamlessly through all interconnected modules—eliminating interface complexities while maintaining impeccable quality and traceability. COSMOS eSource enforces data integrity at the point of entry. These validations improve accuracy, prevent protocol deviations, and meet the electronic Case Report Form (eCRF) guidelines.
Accelerating Site Readiness
Rapid study initiation is an advantage for clinical research sites. COSMOS eSource expedites site readiness by offering centralized source templates, allowing study teams to quickly build and implement their study source data framework. Additionally, automated validation rules enforce data accuracy measures, minimizing discrepancies at the point of entry. This reduces the data quality verification time, ensuring that the captured data is compliant and ready for immediate analysis.
Unlike traditional paper-based systems—where monitors must sift through bulky binders to validate subject data—COSMOS eSource consolidates all relevant trial information into a single, easily navigable digital environment. Investigators, site monitors, and research coordinators can seamlessly review:
- Subject medical records
- Informed consent documentation (ICFs)
- Laboratory results & imaging reports
- Safety event logs (AE, SAE)
- Protocol deviations
Having all data in a single place eliminates the back-and-forth so that teams can monitor progress transparently in real time. Monitors can conduct chart reviews on-site or remotely, ensuring data integration. They can issue queries, track responses, and seamlessly close queries, preventing delays and increasing trial efficiency. Improved data quality leads to reduced monitoring time and fewer visits. Sponsors can optimize their approach by decreasing the number of on-site monitoring visits or increasing remote visits, thereby meeting regulatory compliance while achieving significant cost savings. With eSource, decision-making is quick, allowing study teams to act quickly, rectify discrepancies proactively, and prevent future data inconsistencies, The data can be accessed from anywhere by investigators, enhancing their ability to maintain effective oversight. This digital transformation enhances compliance and reduces protocol deviations, which can increase patient safety by ensuring that trial data aligns with the protocol.
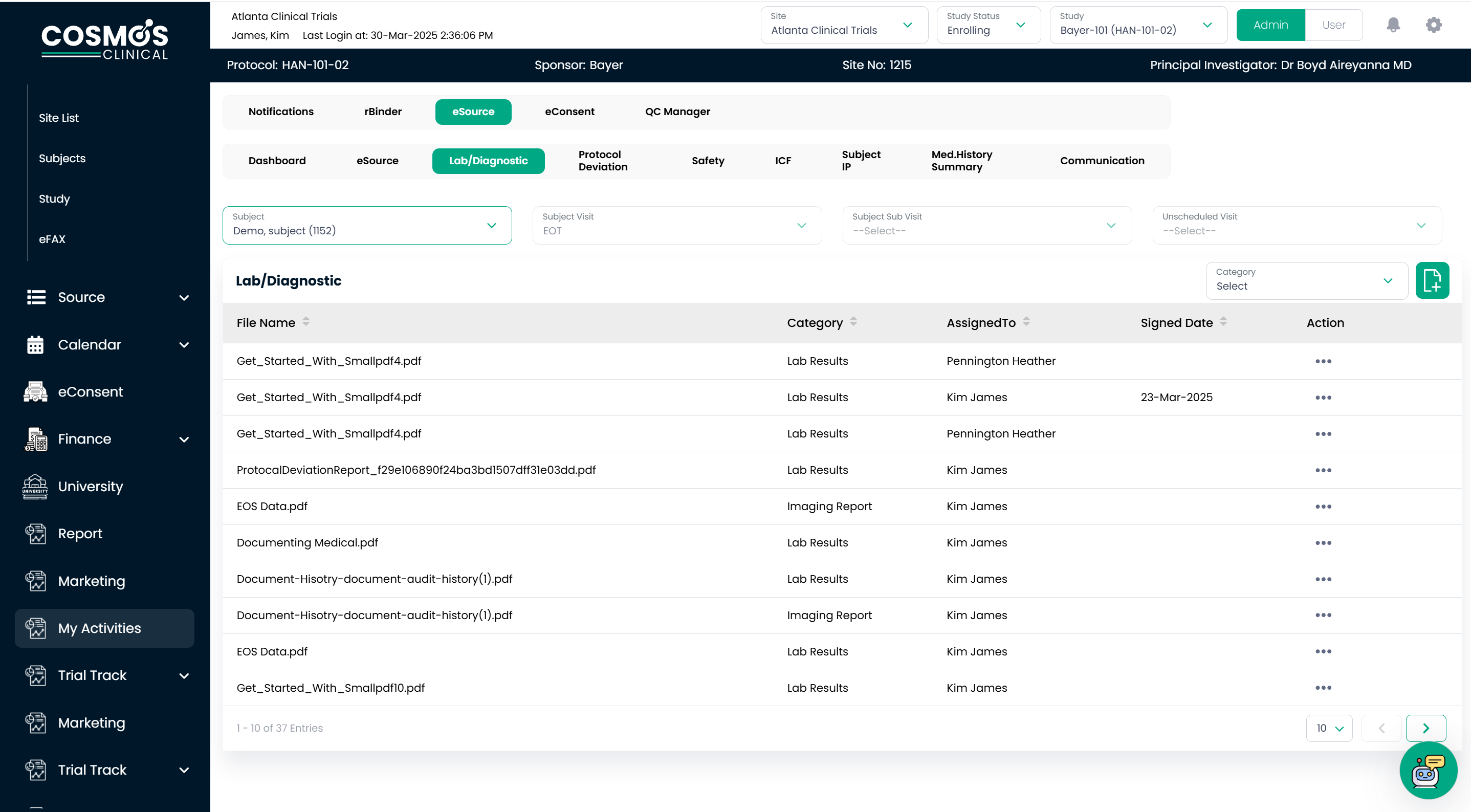
Seamless Lab & Imaging Integration
Clinical data like lab reports and imaging reports are collected from multiple systems, slowing the decision-making process in clinical trials. Most of the time, the site team misplaces the reports received from various systems, which causes delays in the investigator’s subject diagnoses. COSMOS eSource bridges this gap through direct eFax integration with central labs, allowing for the immediate, error-free transmission of:
- Lab reports
- Electrocardiograms (EKG)
- Radiology reports
- Subject Medical Records
By automating the annotation and e-signature workflow, COSMOS eSource ensures that study teams can review, approve, and assign critical findings without delay. Furthermore, all data is housed within a unified digital repository, eliminating interface lags and ensuring that regulatory compliance is maintained without administrative overhead.
Expedited Safety Reporting & Compliance Oversight
Safety in trials lives and dies by how fast and precise adverse event reporting is. But traditional processes? They’re drowning in paperwork: printing forms, chasing signatures, and typing data by hand. This process introduces:
- Delays in regulatory submission
- Higher risk of safety red flags slipping through the cracks
- Extra rounds of manual checks that drain time and focus
- Difficult to follow the ALCOA-C principles
With COSMOS eSource, the entire safety reporting process is digitized and automated. Investigators can instantaneously leverage custom-built AE/SAE templates to generate reports, forwarding them for review and eSing using inline electronic signatures. Real-time alerts provide site monitors with immediate oversight, ensuring that safety data is evaluated without lag. Clear visibility into every step of the process ensures deadlines are met.
The Future of Hybrid & Decentralized Clinical Trials (DCTs)
With the advent of hybrid and decentralized clinical trials, traditional monitoring paradigms are undergoing a radical transformation. COSMOS eSource provides remote access to real-time data and provides site monitors and sponsors to:
- Conduct remote monitoring without frequent on-site visits
- Detect data discrepancies instantly
- Expedite mid-study adjustments based on evolving trial metrics
- Reduce the travel expenses
By eliminating the logistical constraints of paper-based data collection, COSMOS eSource ensures that research sites remain at the forefront of technological evolution—enhancing operational agility while minimizing cost-intensive site visits.
Regulatory Compliance: Simplified, Strengthened, Secured
Regulatory adherence is non-negotiable in clinical trials, and demonstrating compliance is often arduous. COSMOS eSource simplifies this burden by embedding:
- Electronic signatures compliant with FDA 21 CFR Part 11
- Audit trails for full transparency
- Automated version control to prevent data discrepancies
- Role-based access has been implemented to ensure compliance with both blinded and unblinded roles, enhancing data security and regulatory adherence.
During regulatory audits, study teams can quickly retrieve, search, and verify digital records within minutes—compared to the labor-intensive document retrieval process inherent to paper-based trials. This seamless access to comprehensive compliance documentation ensures that study sites remain audit-ready at all times. The site is audit-ready all the time.
A Future-Ready Solution for Clinical Research Sites
COSMOS eSource equips clinical research sites with an intelligent, agile, and highly scalable documentation ecosystem by eliminating redundant manual processes, reducing monitoring costs, and ensuring seamless regulatory alignment.
With its ability to seamlessly integrate with existing workflow, provide real-time monitoring, and maintain data integrity, COSMOS eSource revolutionizes clinical data collection. It empowers research teams to focus on what matters most: advancing medical innovation with precision and efficiency.
EDC integration
With the COSMOS API, eSource seamlessly integrates with Electronic Data Capture (EDC) to grant you access to real-time global study data synced across COSMOS’ fully integrated modules with cloud-based data capture. Enjoy real-time data management, eliminate manual data capture processes, generate rapid real-time reports, and ensure easy data compliance for both research sites and sponsors.
Generate AE/SAE Reports Quickly
Make better decisions within shorter timelines using unified technology. Simply incorporate a custom AE/SAE template into your new AE/SAE dashboard and send it to the investigator for e-signing. Then, automatically email it to the safety team for review. Track all submissions to the research site or investigator remotely and get automated, real-time alerts based on data entry.