How to Comply with the End of Study Documents using COSMOS eVault
A perfect End of Study (EOS) data exchange is critical for the successful closure of a clinical study. Even during a clinical study, it is necessary to maintain a collection of essential documents to be used by the sponsors, CRO, and investigators for the management of the study. It is also helpful to the monitors, auditors, and inspectors to review and verify whether the sponsor and investigator conduct the study in line with the study protocol and regulatory requirements. However, it is tedious to manage and exchange the endless EOS documents from multiple vendors, especially at the end of a study.
COSMOS eVault: End of Study (EOS)
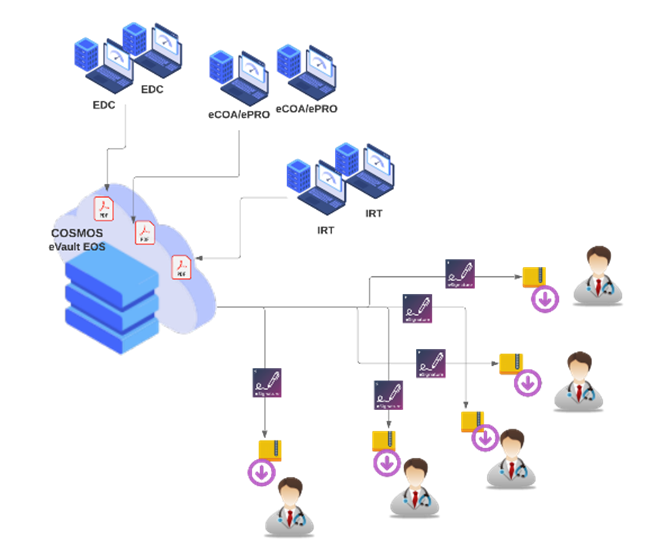
At COSMOS, we have the exact solution for that issue. COSMOS eVault is a customizable and scalable tool used to manage and distribute electronic EOS documents at the end of a study. This unique platform is ideal for digitizing and sharing all EOS documents. It facilitates long-term storage of EOS documents and secure access to them for instant viewing and sharing among authorized users and auditors in the event of inspections and requests. COSMOS eVault eliminates the need to carry out seamless manual paper-based documentation, which is tedious and very time-consuming.
With the COSMOS eVault, sites’ EOS files can be digitized, enabling secure distribution between sponsors and CROs through a unified platform. It also can communicate with multiple vendor systems simultaneously to retrieve the study EOS data. Moreover, it eliminates the need to create and distribute CDs/DVDs/USBs as well as any acknowledgment forms.
There is an artificial intelligence QA process built within the eVault, and once the data arrives at the eVault, it will validate and compress the data before sharing them with the sites. Therefore, every step of the process has been validated before it reaches the next stage. Moreover, the in-built digital signature feature enables the eVault to obtain the site confirmation in real-time. With the use of the platform-independent system, the sponsors can monitor the ongoing progress of the study from anywhere.
Since the system vendors only exchange the data from their systems, data cannot be interfaced with other systems. Currently, it has been necessitated by the sponsors that multiple vendors must be utilized to complete a single task. As COSMOS eVault is a high-breed platform, it will read data from multiple systems for the same study and complete the EOS process.
COSMOS eVault: EOS is the economical solution when it comes to working with multiple product vendors. It also offers the EOS data exchange service for a fraction of the cost compared to the existing options.
Benefits of COSMOS eVault
- Meet the Compliance: COSMOS eVault enables sites to have a secure and controlled access to data with full audit trails. It provides the highest level of information security, data privacy, and quality management.
- Optimize Operational Efficiencies: Cloud-based distribution of EOS documents minimizes the tedious manual exchange of data to the sites through CDs, DVDs, USBs or traditional documents and acknowledgment forms.
- Increase Site Efficiency: COSMOS eVault provides a central location for receiving and distributing EOS documents while complying with the study protocols and regulatory requirements, which takes the burden off from the sites to exchange data efficiently and securely.
- Reduce Distribution Expenditure: Since there is no any physical media like CDs, DVDs, or USBs involved in the process of data management and exchange, eVault reduces the distribution expenditure on EOS documents.
- Optimize Resources Management: The automated process of COSMOS eVault enables an efficient EOS data management and exchange with less resources, eliminating the need for handling physical media.